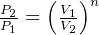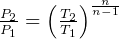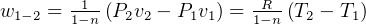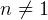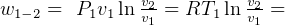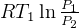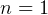up
my study notes, thermodynamics UCI 91, summer 2004
by Nasser M. Abbasi
June 24, 2013
Contents
1 questions
- 8/28/04, 8:20 pm. page 284 in book, the formulas for ds are NOT for polytrpoic process, but for
general ideal gas process (no
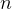 in the formula). So, how to determine
in the formula). So, how to determine 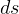 for the 4 different
polytropic processes? In particular how to find
for the 4 different
polytropic processes? In particular how to find 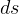 for const T (
for const T (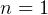 ) process? The other book
has it, but do not see it now in our text book.
) process? The other book
has it, but do not see it now in our text book.
- page 284, equation for
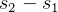 for ideal gas, why does book write
for ideal gas, why does book write 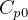 but only
but only 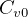 for the next
equation below it? Should not they both be for ideal gas, i.e. with a ‘
for the next
equation below it? Should not they both be for ideal gas, i.e. with a ‘ ’ in the subscript?
’ in the subscript?
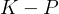 statment of second law basically says that it is impossible to reach
statment of second law basically says that it is impossible to reach 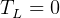 , right? becuase
when
, right? becuase
when 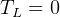 then
then 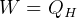 for an ideal cycle.
for an ideal cycle.
- 8/28/04, 9:20 pm. What about shaft work for non-steady state flow? Do not need to worry about
it in this course.
verify what it will be for 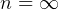 , ie. Constant
, ie. Constant 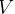
- Since Gibbs equations were derived assuming a reversible process and compressible substance, then I
assume we can’t use these for solids/liquids? Verify.
- How do we derive
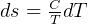 for solids?, I see we can get it Gibbs equation by setting
for solids?, I see we can get it Gibbs equation by setting 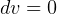 for
solids, But Gibbs equations were derived for incompressible substances??
for
solids, But Gibbs equations were derived for incompressible substances??
answer: do not use Gibbs. always start from basic laws to be safe. use entropy equation 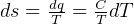 .
simple.
.
simple.
- In deriving the entropy change equation for ideal gas, the book for some reason ignores the term
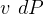 ,
why?? see page 263. see my derivation below
,
why?? see page 263. see my derivation below
- Why is
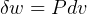 only and not
only and not 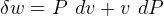
2 To investigate
Model carnot cycle as water flow and using flywheel. Then translate the 2nd law statments to this
model.
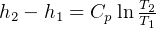
entropy change for ideal gas:
but 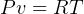 , hence
, hence
entropy change for Solids/liquids:
but 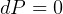 since incompressible,
since incompressible,
and 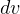 is very small, so
is very small, so
Now, for constant 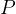 we get from equation (1)
we get from equation (1)
But 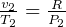 and
and 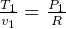 so
so
so, for constant P, for ideal gas
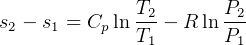
but if constant P, then 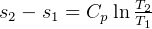 ??
??
Process that causes irreversibility
- Friction
- Unrestrained expansion
- Heat transfer from hot to cold body
- Mixing of 2 differrent substances
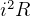 loss in electric circuits
loss in electric circuits
- Hystereris effects
- Ordinary combustion
First Law, non-flow
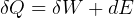
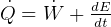
where 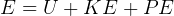
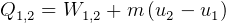
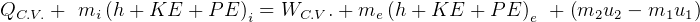
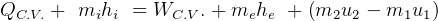
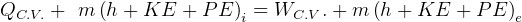
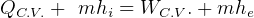
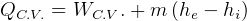
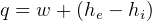
steady state. 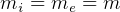
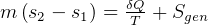
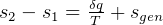
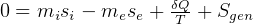
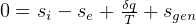
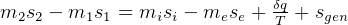
3 Chapter 5. First law of thermodynamics
This law is the conservation of energy law.
It says that change of internal energy of a system equals the difference between the heat energy entering the
system and the work produced by the system.
In symbols, let 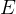 be the energy of the control mass or volume,
be the energy of the control mass or volume, 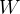 , work produced,
, work produced, 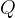 is the heat energy
gained. Then
is the heat energy
gained. Then
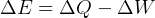
Usually we have a process from one state to the next, so the above is written as
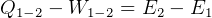
energy is 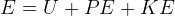
enthalpy
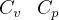
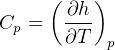
3.1 Solids and liquids equations
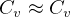 For solids and liquids
For solids and liquids
Now, by definition, 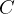 is the heat energy
is the heat energy
to raise
Hence for solids/liquids, 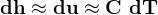
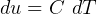
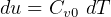
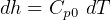
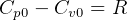
For solids and liquids, 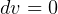 since almost incompressible, hence
since almost incompressible, hence
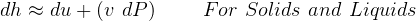
Also for solids and liquids, 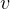 is very small, hence
is very small, hence
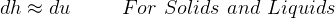
3.2 Signs for energy and work
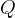 in is
in is 
 out is
out is 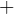
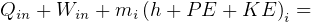
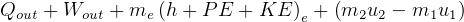
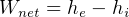
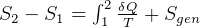
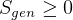
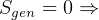 reversible process
reversible process
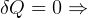 adiabatic process
adiabatic process
Hence, 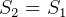 for a reversible adiabatic process
for a reversible adiabatic process
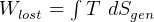
Actual boundary work 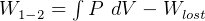
4 Gibbs relations
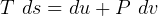
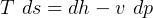
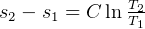
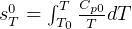
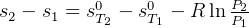 Using table A.7 or A.8
Using table A.7 or A.8
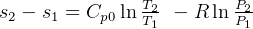 Constant
Constant 
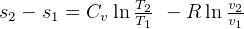 Constant
Constant 
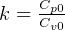
5 Polytrpic process
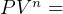 constant
constant
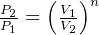
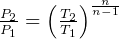
specific work (work is moving boundary work, 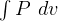
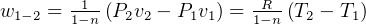
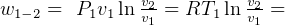
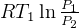
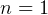
6 study agenda notes
6.1 Monday August 30, 2004
12 midnight. Working on derivation of the entropy change equations. Putting the questions I have into separate
file (pdf, html).
Spend more time going over the conceptual questions for each chapter. These are good.finished chp 8. now
doing chp 9.
3:10 AM. Done. Finished chp9 conceptual questions, and went over 1st and 2nd laws and few things.
Updated the diagram. Now at 11x17 paper size, need to figure how/where to print it.
6.2 Sunday August 29, 2004
3:40 PM. Sitting down to try to do some studying. Where does time go?
6.3 Saturday August 28, 2004
Working on this diagram , which does better classifications of the different processes involved. I am finding it
hard to put everything in one place, because we have 2 main classifications, Gasses and Solids/liquids, and
then we have to classify based on the process type (including ideal vs not ideal cases for Gasses), then we
have the reversible vs. irreversible process to classify on, and then we have the flow vs non-flow
process.
doing this diagram is helping me better figure where to use which law than I did before.
Got my final grade not including the final exam, which is 53.6/60, this is 89.4%.
Since finals has 40 points, then I need to get 40/40 in the finals to get 93.6% final grade to get a chance of
an A. Not sure I can get it. So may be IŠll get an A- or a B+.
I have decided that our text book is not well organized. The author does not classify different things using
tables or diagrams.
I am now studying from text book called ŚElements of applied thermodynamicsŠ by Johnston and
Brockett. I really like the polytropic process discussion there and how they show the different cases for different
n values. Much clearer than our textbook.
10:43 PM. Just learned a cool trick to remember the P-v and T-s diagrams. They all are clock-wise
0,1,k,infinity. For P-v, draw the straight lines first (const P, n=0) and const v (n=infinity). Then using the
clock-wise direction the rest follow. For T-s diagram, again draw the straight lines, const T (n=1) and const s
(n=k), and the rest follows.
Wish I learned this before that quiz which asked about this!
If a process is irreversible, then only end points are known. So use a dotted line to draw such a process
on P-v or T-s. reversible processes are known at each point between the end states. Use solid
line.
Area under irreversible process on P-v or T-s diagram has NO SIGNAIFANCE.
Only area under reversible process. For a reversible process, area under T-s is the heat energy, while under
the P-v is the work.
2nd law statements: No engine, actual or ideal, when operating in a cycle, can convert all the heat supplied
to it into mechanical work.
Clausius statement: "it is impossible for a self-acting machine, unaided by an external agency, to transfer
heat continuously from one body to another at a higher temp".
Basically this says that work is needed to force heat to travel from a lower temp body to a higher temp
body.
See if I can get the Canot book "Reflections on the motive power of heat" on amazon. Originally French,
may be there is a translation.
Carnot principle: "The motive power of heat is independent of the agents employed to realize it; its
quantity is fixed solely by the temp. of the bodies between which is effected, finally, the transfer of
heat"
The efficiency of a reversible heat engine cycle depends only of the temp. of the heat energy source and
sink.
1:20 AM. Go to sleep. Tomorrow need to start working on solving actual problems.
6.4 Sunday August 22, 2004
11:30 PM, Working on last HW. 4 problems done, this HW is taking long time.
Spend time doing this visio diagram, was getting lost with all the relations and not being clear when to use
which under what conditions. Still can not see my grades by looking at e3.uci.edu, a bug in the
system.
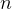 in the formula). So, how to determine
in the formula). So, how to determine 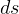 for the 4 different
polytropic processes? In particular how to find
for the 4 different
polytropic processes? In particular how to find 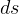 for const T (
for const T (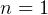 ) process? The other book
has it, but do not see it now in our text book.
) process? The other book
has it, but do not see it now in our text book.
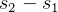 for ideal gas, why does book write
for ideal gas, why does book write 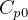 but only
but only 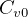 for the next
equation below it? Should not they both be for ideal gas, i.e. with a ‘
for the next
equation below it? Should not they both be for ideal gas, i.e. with a ‘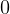 ’ in the subscript?
’ in the subscript?
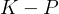 statment of second law basically says that it is impossible to reach
statment of second law basically says that it is impossible to reach 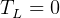 , right? becuase
when
, right? becuase
when 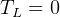 then
then 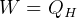 for an ideal cycle.
for an ideal cycle.
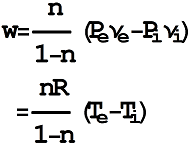
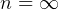 , ie. Constant
, ie. Constant 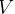
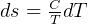 for solids?, I see we can get it Gibbs equation by setting
for solids?, I see we can get it Gibbs equation by setting 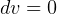 for
solids, But Gibbs equations were derived for incompressible substances??
for
solids, But Gibbs equations were derived for incompressible substances??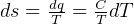 .
simple.
.
simple.
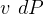 ,
why?? see page 263. see my derivation below
,
why?? see page 263. see my derivation below
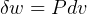 only and not
only and not 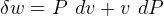
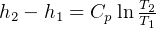
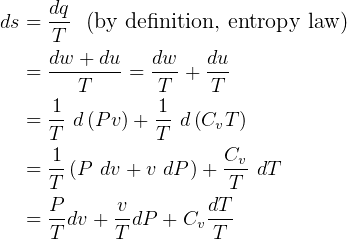
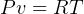 , hence
, hence
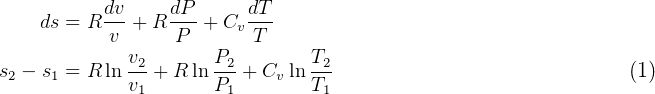
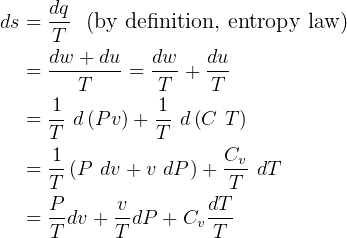
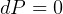 since incompressible,
since incompressible,
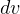 is very small, so
is very small, so

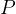 we get from equation (1)
we get from equation (1)
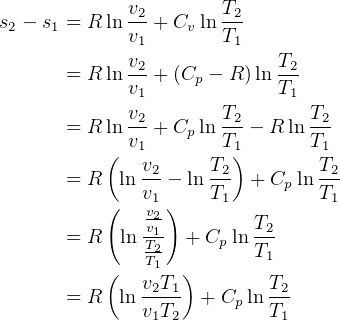
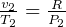 and
and 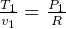 so
so
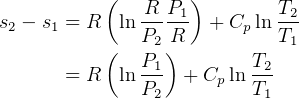
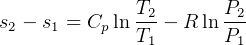
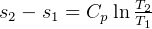 ??
??
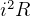 loss in electric circuits
loss in electric circuits
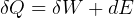
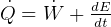
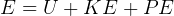
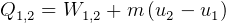
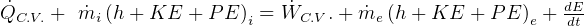
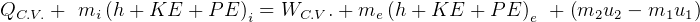
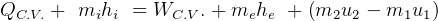
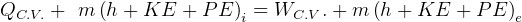
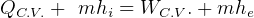
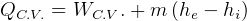
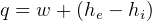
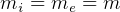
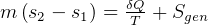
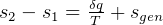
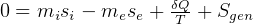
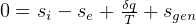
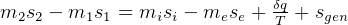
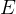 be the energy of the control mass or volume,
be the energy of the control mass or volume, 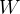 , work produced,
, work produced, 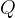 is the heat energy
gained. Then
is the heat energy
gained. Then
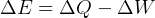
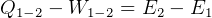
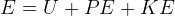
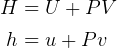
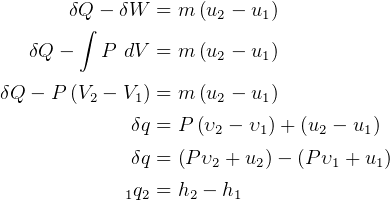
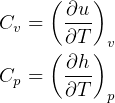
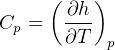
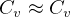 For solids and liquids
For solids and liquids
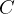 is the heat energy
is the heat energy
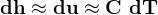
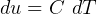
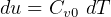
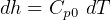
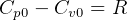
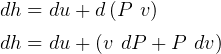
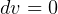 since almost incompressible, hence
since almost incompressible, hence
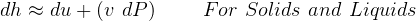
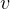 is very small, hence
is very small, hence
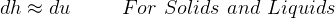
 in is
in is 
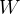 out is
out is 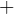
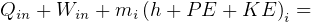
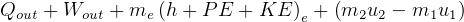
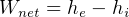
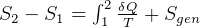
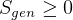
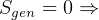 reversible process
reversible process
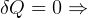 adiabatic process
adiabatic process
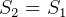 for a reversible adiabatic process
for a reversible adiabatic process
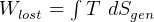
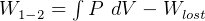
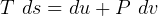
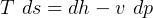
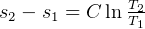
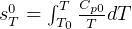
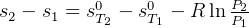 Using table A.7 or A.8
Using table A.7 or A.8
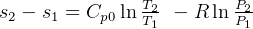 Constant
Constant 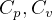
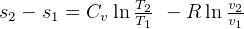 Constant
Constant 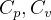
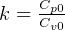
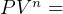 constant
constant