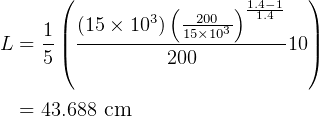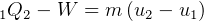
see book.
air is ideal gas
constant specific heat coefficients
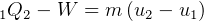
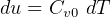
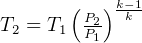 for adiabatic process
for adiabatic process
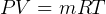
Control mass energy equation gives
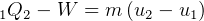
Since adiabatic, then 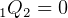 , hence
, hence
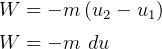
But 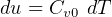 , hence above becomes
, hence above becomes
Need to find 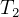
Since adiabatic, then
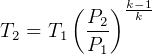
Where 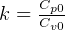
So equation (1) becomes
Mass 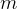 can be found from ideal gas law.
can be found from ideal gas law. 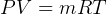 , hence
, hence 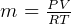 , so equation (2) becomes
, so equation (2) becomes
For air, from table A.5, use 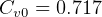 KJ/Kg-K,
KJ/Kg-K,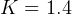 ,
, 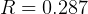 , so equation (3) becomes
, so equation (3) becomes
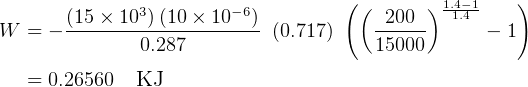
NOw To find the length.
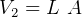
Hence
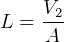
But 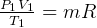 and
and 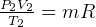 , so
, so 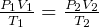 , hence
, hence 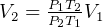
So
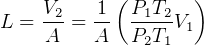
but 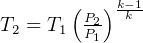 hence
hence
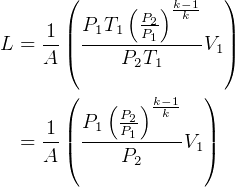
Given 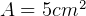 ,
, 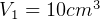 so
so