up
study notes, physics 7D, UCI summer 2003
by Nasser M. Abbasi
June 24, 2013
Constants to know
- permitivity in vacume
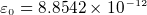 Nm
Nm /C
/C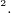 related is columb constant,
related is columb constant, 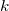 , which is
, which is 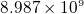 Nm
Nm /C
/C
- gravitional constant
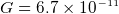 Nm
Nm /kg
/kg
- electron charge
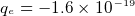 C
C
- electron mass
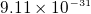 kg
kg
- proton mass
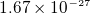 kg
kg
- resistivity
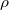 . copper=
. copper=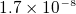 ohm meter. see page 847.
ohm meter. see page 847.
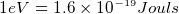
- one mole contains avegadro number of atoms=
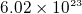 . need to be given the weight of a mole of a substance. If
given density, then we can find number of electrons per one meter cubic. use it in the equation
. need to be given the weight of a mole of a substance. If
given density, then we can find number of electrons per one meter cubic. use it in the equation 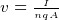
1 Things to know
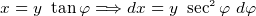
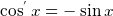
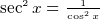
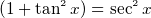
2 Notes
To move a point charge from one point A to another point B, the field E must do some amount of work. Since there is no free luch
in life, there is an energy exchange. (conservation of energy). The energy needed to move the charge comes from the loss of the
charge potional energy.
Hence this is why the potional energy of the charge is reduced by the amount of work it has done. This is why we put a minus
sign in this express 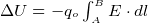
We can only talk about potential energy differences and electric potential differences. The difference between 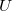 and
and 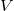 is
that
is
that 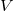 is the energy difference PER UNIT charge.
is the energy difference PER UNIT charge.
3 Definitions
- Electric field E is force per unit charge. Has units of Newton per Columb. To get a feel for E, a lamp will produce an
E of about 10 N/C, and a baloon rubbed on hair will produce an E of 1,000 N/C. Since there are about
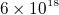 electrons to make one columb of charge, then a lamp will cause a force of 10 N on those electrons, or a force of
about
electrons to make one columb of charge, then a lamp will cause a force of 10 N on those electrons, or a force of
about 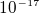 Newton on each electron. Now, since E near an electron in a hydrogen atom is about
Newton on each electron. Now, since E near an electron in a hydrogen atom is about 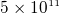 N/C,
then the force on an electron in a hydrogen atom is about
N/C,
then the force on an electron in a hydrogen atom is about 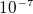 N, or it is about
N, or it is about 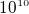 as large as the force produce
outside a lamp. This shows that at atomic scale, the columb forces are much greater, this is due to the much smaller
distance, and the
as large as the force produce
outside a lamp. This shows that at atomic scale, the columb forces are much greater, this is due to the much smaller
distance, and the 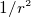 term in coulmb law.
term in coulmb law.
- When we decrease distance between capcitor plates, charge on each plate increaes, hence capacitance increases. But
voltage across the plates is always the same as the battery voltage.
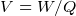 , i.e. Volt is work per unit charge. i.e.
, i.e. Volt is work per unit charge. i.e. 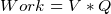
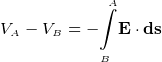
- For a point charges
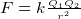
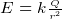
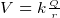
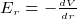 so if we know
so if we know 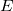 we can find
we can find 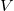 by integration
by integration
- Capacitance formulas
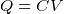
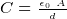
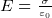 where
where 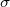 is the charge density on the capictor plate
is the charge density on the capictor plate 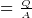
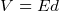
potential energy stored in capacitor 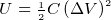
capacitance of a sphere with radius  and charge
and charge 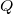 is
is 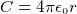
Energy stored in a capacitor = 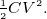
Energy per unit volume inside a capacitor is 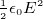
- Area of sphere=
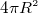
- Circumferance of circle =
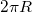
- area of circle =
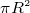
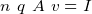 , note that here
, note that here 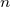 is the number of electrons per square meter! , so to find this value, one needs to
know the density, and the molar mass and avegadro number. Once
is the number of electrons per square meter! , so to find this value, one needs to
know the density, and the molar mass and avegadro number. Once 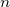 is found, then speed
is found, then speed  is easily found since
is easily found since
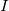 is given.
is given.
- Current density
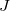 , is current per unit area. Hence
, is current per unit area. Hence 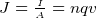
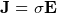 , this is OHM’s law.
, this is OHM’s law. 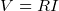 , where
, where 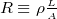 , where
, where 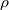 is the resistivity, which is ohms per meter.
is the resistivity, which is ohms per meter.
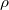 is defined as
is defined as 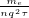 where
where  is the average time between collisions.
is the average time between collisions. 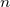 is the number of electrons per meter
cubic.
is the number of electrons per meter
cubic.
- power supplied to a resistor by a battery is
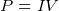 or
or 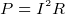
- Remember, if given the battary internal resistance, then add it to the external resistance to get the total resistance.
4 Typical values of things
- the Earth magnetic field is typically around 500 mG.
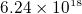 electrons make up one Columb of negative charge.
electrons make up one Columb of negative charge.
- One cubic cm of copper contains about
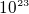 electrons. So one cubic cm of copper contains about
electrons. So one cubic cm of copper contains about 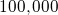 of
negative columb charge.
of
negative columb charge.
- In typical rubbbing of glass by silk, only about a total
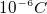 of charge is transfered, this is about
of charge is transfered, this is about 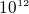 electrons
being transfered, or about the number of electrons inside a micro cubic cm.
electrons
being transfered, or about the number of electrons inside a micro cubic cm.
5 What is a spectrometer
(spectrograph, quantometer, spark emission, optical emission, spec, OES, ICP, plasma, spectro analyzer)?
A spectrometer system is a device that vaporizes material in a plasma discharge, either by electrical sparking for metallic
samples, or by a sustained plasma(ICP) for fluids to generate light which emits spectral information on the elemental
concentration of the sample. The spectrometer measures the light energy of several wavelengths and converts the light energy to
electrical current, where it is measured or digitized by electronics and applied to calibration curves stored in the
operating software. Analysis times are less than 1 minute. The printout display shows sample I.D.’s, alloy names, and
elemental concentration for each element in % concentration or PPM. Accuracy is outstanding and detection limits are
PPB.
6 On spark in air and voltage
from the net
The electric field required to cause sparks through air is roughly 30
KV per centimeter.
In any situations where the electric field is not evenly distributed
between the electrodes, the air can break down in a part of the gap
between the electrodes. The ionized air is conductive enough to
facilitate breakdown, or ionization, of the remaining portion. Between
sharp points, the voltage required to cause a spark is about 11 KV per
centimeter at most voltages from 5 to 40 KV. At higher voltages, lower
voltages per centimeter can cause sparking.
The voltage required to generate a spark varies roughly inversely with
the density of the air. Therefore, this voltage varies roughly directly
with barometric pressure and roughly inversely with absolute temperature.
However, as to humidity....
Even on a bad summer day in southern, eastern, or midwestern parts of
the USA....
"Tropically" humid air is generally 3 to 4 percent water vapor, and 96
to 97 percent of the gases normally found in air. I believe this usually
does not make much difference.
Just beware that some normally insulating substances will absorb water
from air if the relative humidity is high. This can affect the behavior
of the insulators at higher voltages. This sometimes affects the nature
of sparks, coronas, and other discharges from points at or near where
conductors meet humidity-sensitive insulators. Also, dust that settles
on insulators may contain salt or other humidity-sensitive substances.
This may cause humidity-dependent performance of insulators.
This effect varies widely with amount and nature of any dust, type and
grade of the insulator, etc. etc. Such info will probably not be found
in general tables.
For more specific information about spark-gap voltages and how they
vary with pressure and temperature, see the spark-gap voltage table in
the Handbook of Chemistry and Physics, published by the Chemical Rubber
Publishing Co. Sorry, this table does not quantitavely mention the
effects of humidity.
- Don Klipstein (Jr) (don@misty.com or klipstei@netaxs.com)
7 Paschen’s Law
from the net
In 1889, F. Pashchen published a paper ( Wied. Ann., 37, 69)
which set out what has become known as Paschen’s Law. The
law essentially states that the breakdown characteristics
of a gap are a function (generally not linear) of the product
of the gas pressure and the gap length, usually written
as V= f( pd ), where p is the pressure and d is the gap distance.
In actuality, the pressure should be replaced by the gas density.
For air, and gaps on the order of a millimeter, the breakdown is
roughly a linear function of the gap length: V = 30pd + 1.35 kV,
where d is in centimeters, and p is in atmospheres.
8 references
The internet, wiki
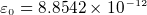 Nm
Nm /C
/C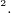 related is columb constant,
related is columb constant, 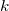 , which is
, which is 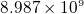 Nm
Nm /C
/C
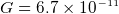 Nm
Nm /kg
/kg
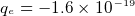 C
C
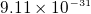 kg
kg
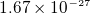 kg
kg
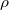 . copper=
. copper=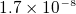 ohm meter. see page 847.
ohm meter. see page 847.
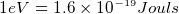
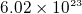 . need to be given the weight of a mole of a substance. If
given density, then we can find number of electrons per one meter cubic. use it in the equation
. need to be given the weight of a mole of a substance. If
given density, then we can find number of electrons per one meter cubic. use it in the equation 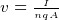
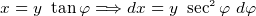
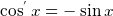
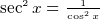
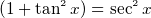
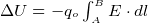
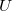 and
and 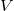 is
that
is
that 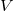 is the energy difference PER UNIT charge.
is the energy difference PER UNIT charge.
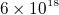 electrons to make one columb of charge, then a lamp will cause a force of 10 N on those electrons, or a force of
about
electrons to make one columb of charge, then a lamp will cause a force of 10 N on those electrons, or a force of
about 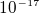 Newton on each electron. Now, since E near an electron in a hydrogen atom is about
Newton on each electron. Now, since E near an electron in a hydrogen atom is about 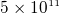 N/C,
then the force on an electron in a hydrogen atom is about
N/C,
then the force on an electron in a hydrogen atom is about 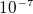 N, or it is about
N, or it is about 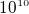 as large as the force produce
outside a lamp. This shows that at atomic scale, the columb forces are much greater, this is due to the much smaller
distance, and the
as large as the force produce
outside a lamp. This shows that at atomic scale, the columb forces are much greater, this is due to the much smaller
distance, and the 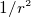 term in coulmb law.
term in coulmb law.
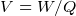 , i.e. Volt is work per unit charge. i.e.
, i.e. Volt is work per unit charge. i.e. 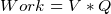
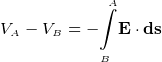
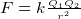
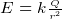
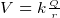
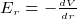 so if we know
so if we know 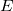 we can find
we can find 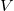 by integration
by integration
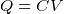
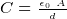
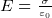 where
where 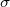 is the charge density on the capictor plate
is the charge density on the capictor plate 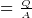
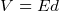
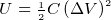
 and charge
and charge 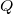 is
is 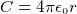
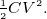
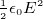
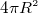
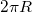
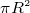
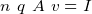 , note that here
, note that here 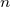 is the number of electrons per square meter! , so to find this value, one needs to
know the density, and the molar mass and avegadro number. Once
is the number of electrons per square meter! , so to find this value, one needs to
know the density, and the molar mass and avegadro number. Once 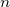 is found, then speed
is found, then speed  is easily found since
is easily found since
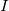 is given.
is given.
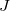 , is current per unit area. Hence
, is current per unit area. Hence 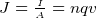
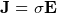 , this is OHM’s law.
, this is OHM’s law. 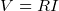 , where
, where 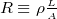 , where
, where 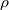 is the resistivity, which is ohms per meter.
is the resistivity, which is ohms per meter.
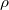 is defined as
is defined as 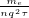 where
where  is the average time between collisions.
is the average time between collisions. 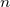 is the number of electrons per meter
cubic.
is the number of electrons per meter
cubic.
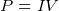 or
or 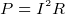
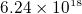 electrons make up one Columb of negative charge.
electrons make up one Columb of negative charge.
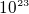 electrons. So one cubic cm of copper contains about
electrons. So one cubic cm of copper contains about 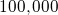 of
negative columb charge.
of
negative columb charge.
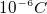 of charge is transfered, this is about
of charge is transfered, this is about 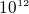 electrons
being transfered, or about the number of electrons inside a micro cubic cm.
electrons
being transfered, or about the number of electrons inside a micro cubic cm.